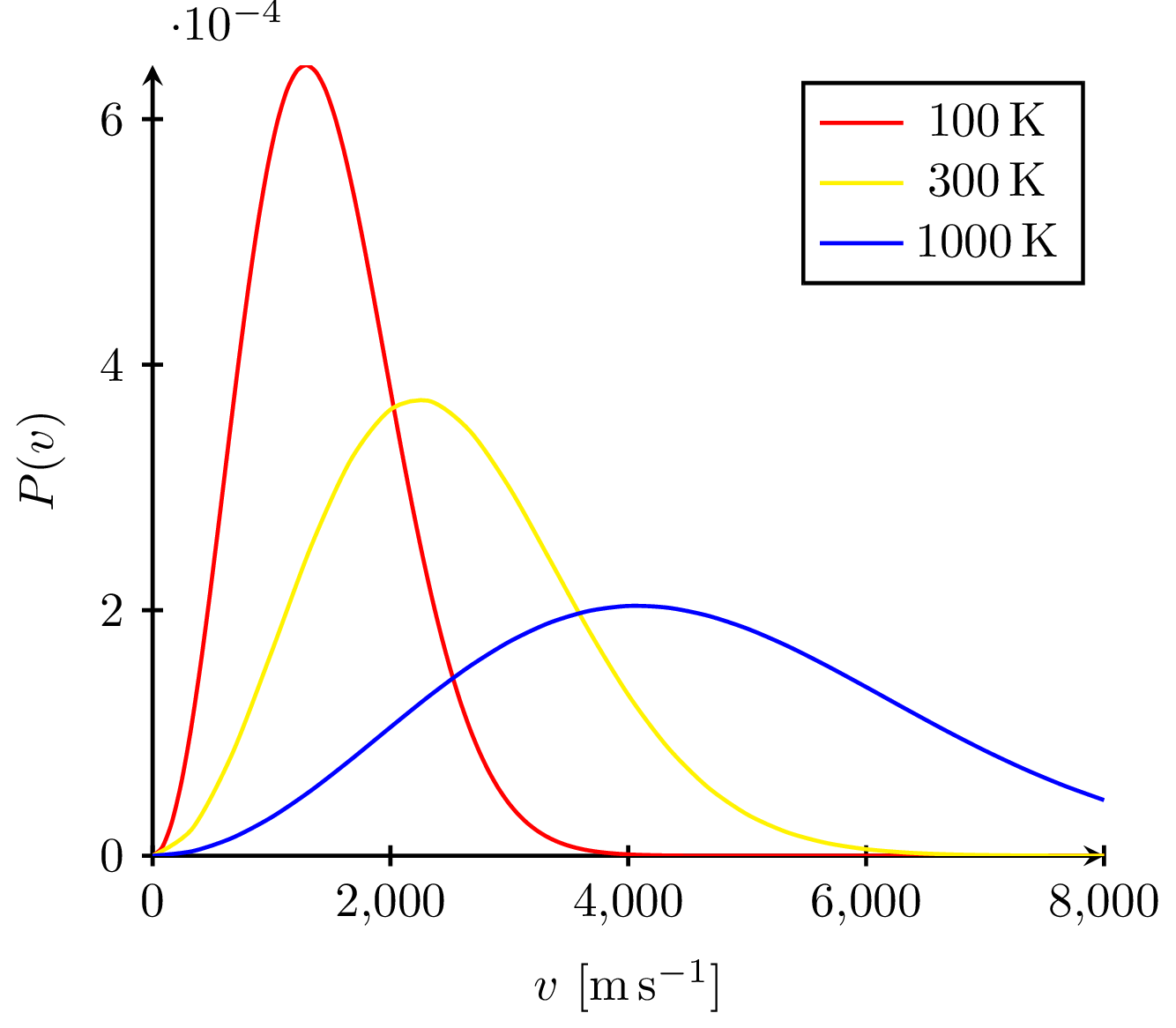
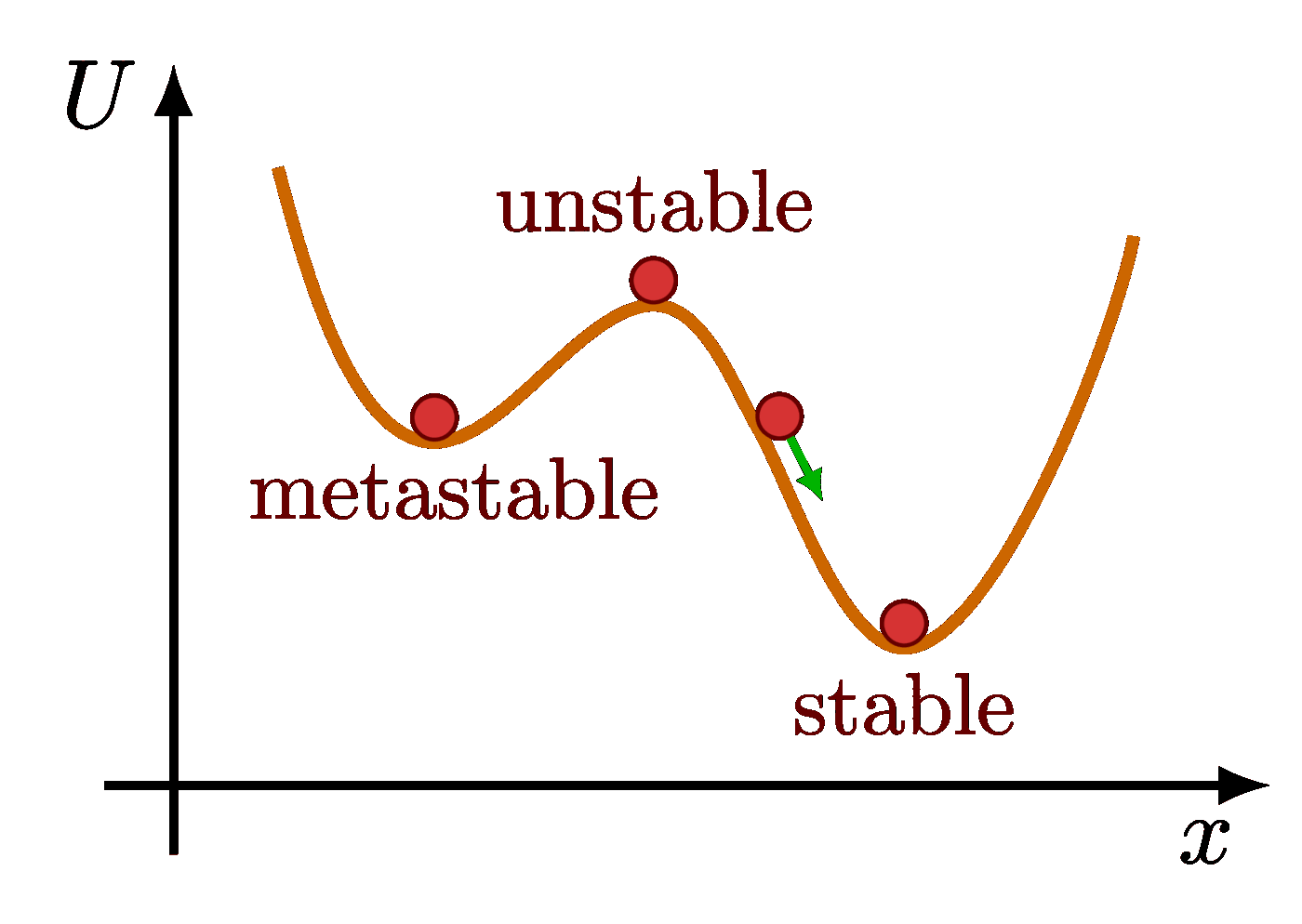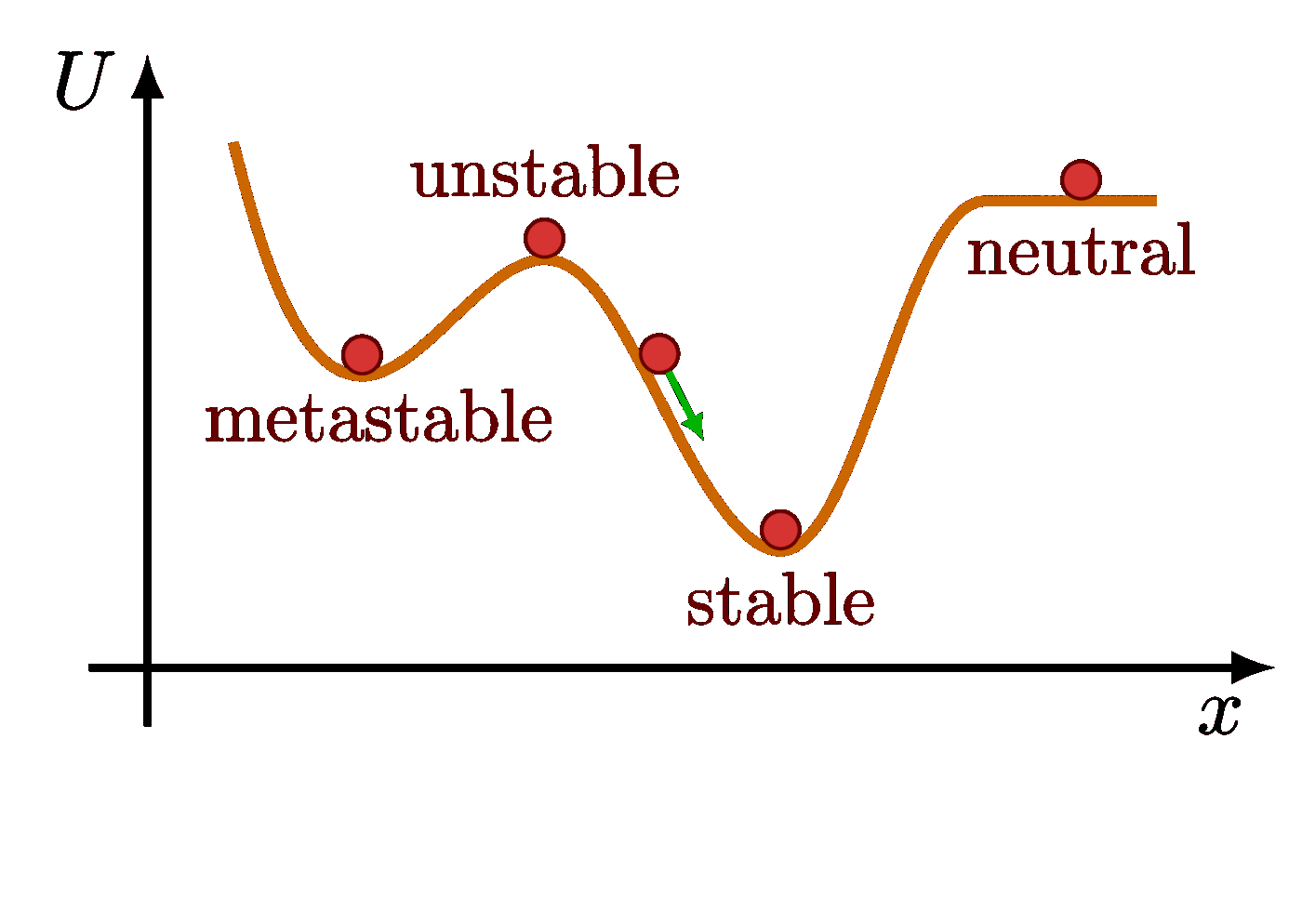
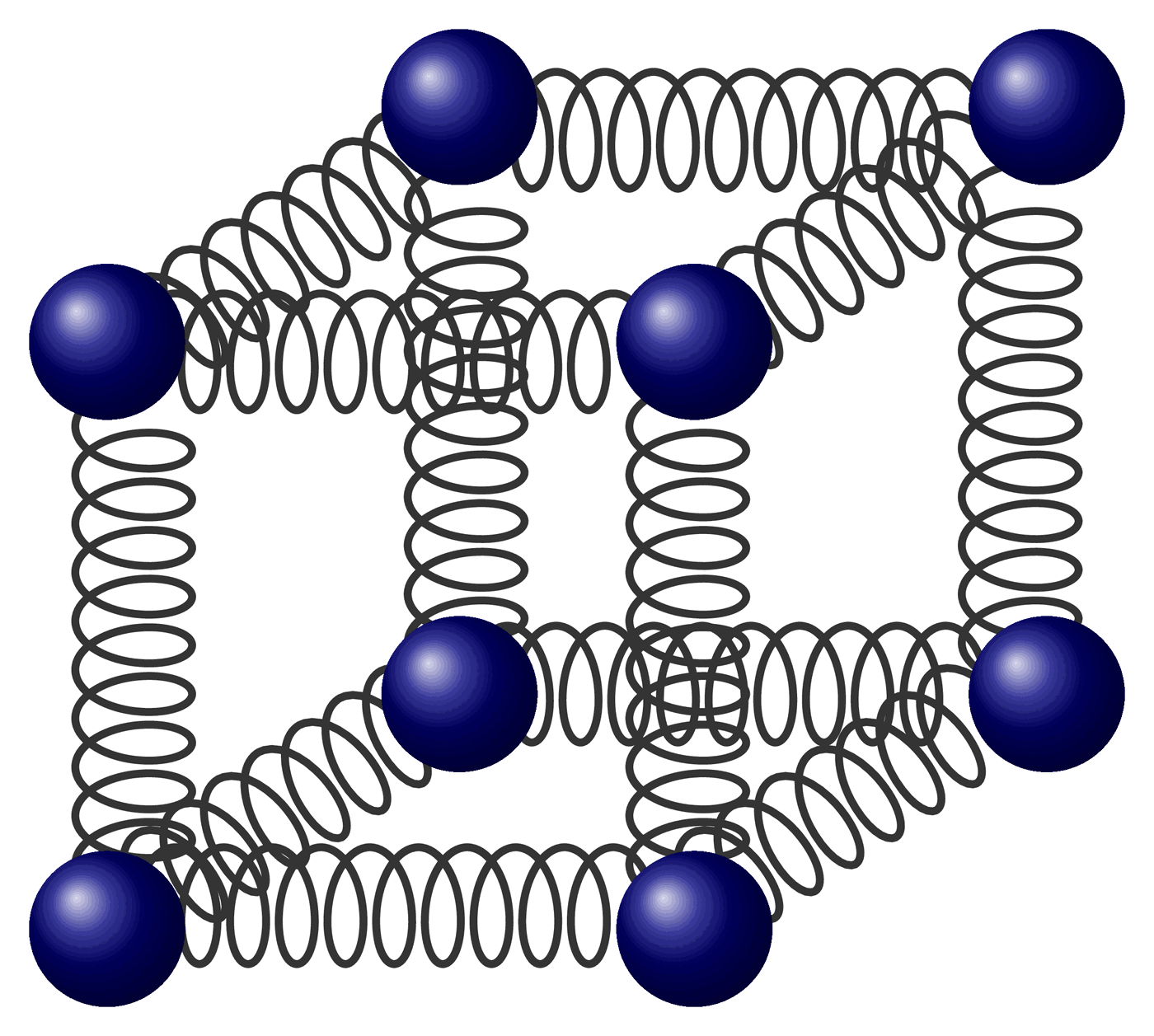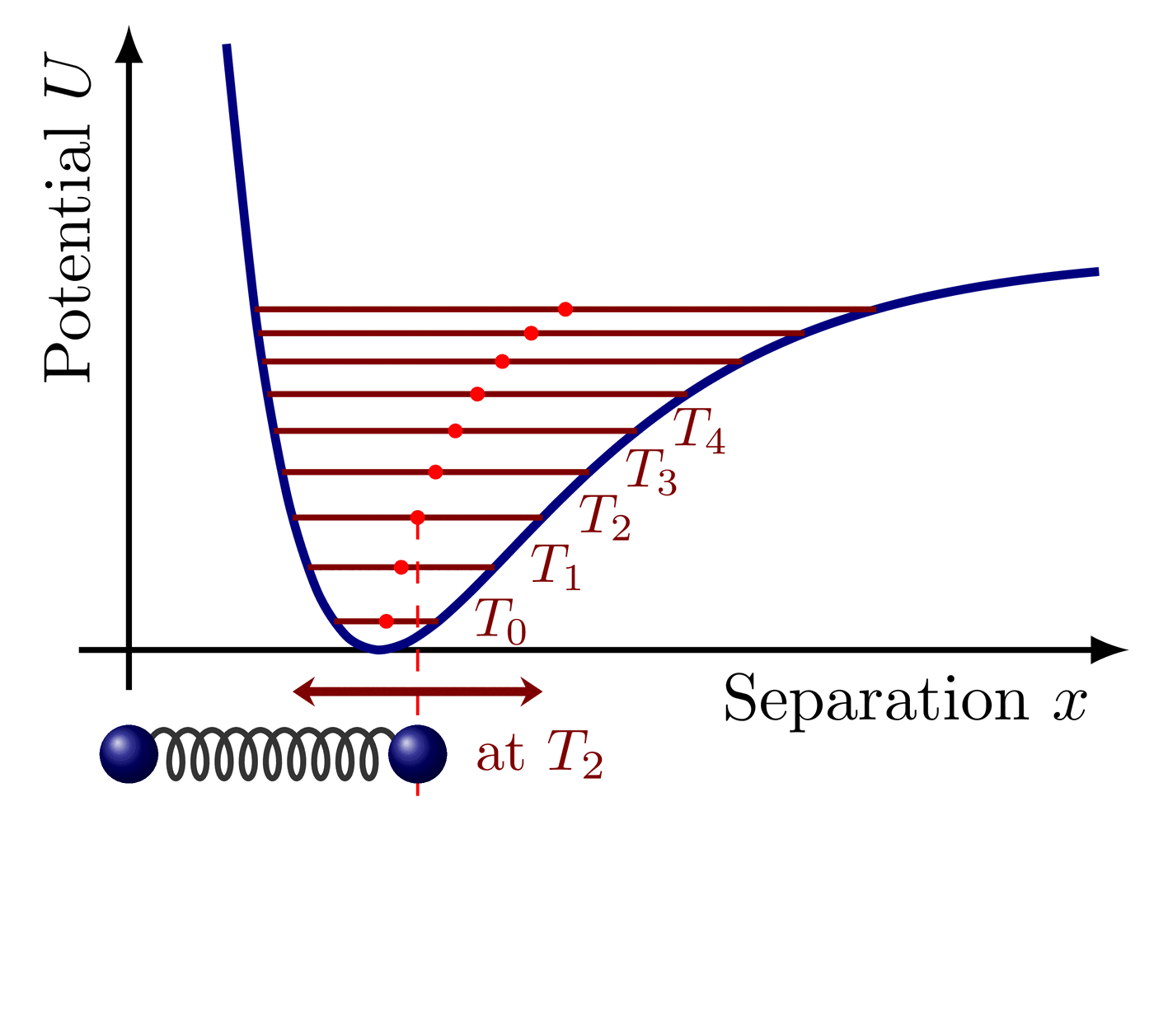
The Maxwell-Boltzmann distribution plotted at different temperatures reveals that the most probable velocity $v_p$ of ideal gas particles scales with the square root of temperature.
Edit and compile if you like:
% The Maxwell-Boltzmann distribution plotted at different temperatures reveals that the most probable velocity v_p of ideal gas particles scales with the square root of temperature.
\documentclass{standalone}
\usepackage{pgfplots,siunitx}
\pgfplotsset{compat=newest}
\def\kB{1.38e-23} % Boltzmann constant
\def\mu{1.66e-27} % unified atomic mass unit/Dalton (symbols: m_u or Da)
\def\maxwellboltzmann#1{4 * pi * (\mu / (2 * pi * \kB * #1))^(3/2) * x^2 * exp(-\mu * x^2 / (2 * \kB * #1))}
\begin{document}
\begin{tikzpicture}
\begin{axis}[
domain = 0:8000,
xlabel = {$v$ [\si{\metre\per\second}]},
ylabel = $P(v)$,
smooth, thick,
axis lines = left,
every tick/.style = {thick},
]
\addplot[color=red,samples=100]{\maxwellboltzmann{100}};
\addplot[color=yellow]{\maxwellboltzmann{300}};
\addplot[color=blue]{\maxwellboltzmann{1000}};
\legend{\SI{100}{\kelvin}, \SI{300}{\kelvin}, \SI{1000}{\kelvin}}
\end{axis}
\end{tikzpicture}
\end{document}
Click to download: maxwell-boltzmann-dist.tex
Open in Overleaf: maxwell-boltzmann-dist.tex
This file is available on tikz.netlify.app and on GitHub and is MIT licensed.
See more on the author page of Janosh Riebesell..